The Hinge Region Strengthens the Nonspecific Interaction between Lac-Repressor and DNA: A Computer Simulation Study
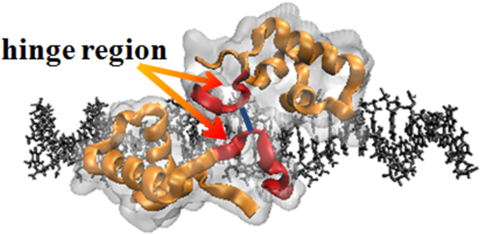
LacI is commonly used as a model to study the protein-DNA interaction and gene regulation. The headpiece of the lac-repressor (LacI) protein is an ideal system for investigation of nonspecific binding of the whole LacI protein to DNA. The hinge region of the headpiece has been known to play a key role in the specific binding of LacI to DNA, whereas its role in nonspecific binding process has not been elucidated. Here, we report the results of explicit solvent molecular dynamics simulation and continuum electrostatic calculations suggesting that the hinge region strengthens the nonspecific interaction, accounting for up to 50% of the micro-dissociation free energy of LacI from DNA. Consequently, the rate of microscopic dissociation of LacI from DNA is reduced by 2~3 orders of magnitude in the absence of the hinge region. We find the hinge region makes an important contribution to the electrostatic energy, the salt dependence of electrostatic energy, and the number of salt ions excluded from binding of the LacI-DNA complex.